
YCP in Numbers

Navigating the EU medical device sector means understanding MDR compliance, as
74%
of applications faced delays in 2024*, highlighting the need for strategic market entry.
Source: MedTech Europe, 2024
YCP Medical Device Experts in Europe
Europe boasts a flourishing landscape in the medical device industry, marked by rapid advancements. The region is blooming with innovations across sectors, prominently in the medical device sphere, encouraging robust growth.
YCP leverages its global expertise and profound understanding to deliver outstanding medical device consulting, capable of tackling Europe's intricate market barriers. This prowess aids clients in smoothing entry and nurturing growth in this dynamic domain.
YCP emerges as a reliable consulting partner for businesses aspiring to excel in Europe's medical device market. Our strategic insights ensure businesses meet their objectives with confidence and drive in the competitive landscape.
Unlocking Potential: Your Path to Excellence with
Europe's Medical Device Professionals
Go to Market Strategy
YCP excels in Europe, offering expert guidance and strategic insights to propel success in the competitive medical device market.
Channel Optimization
Harnessing unmatched European insights, YCP excellently optimizes distribution channels, thus amplifying medical device success and market presence.
Product Market Fit
With comprehensive expertise, YCP guides medical device companies in Europe towards optimal product market fit by leveraging unique industry insights.
Regulatory Access Planning
Navigating the stringent regulatory landscape, YCP's expertise in Europe medical device consulting ensures seamless compliance and strategic market access.
Our Services
Marketing & Sales
Propelling European medical device firms through strategic marketing innovation and robust sales strategies.
- Branding & Brand Management
- Product & Portfolio Management
- Pricing
- Digital Marketing
- E-commerce
- Customer Experience
- Sales Channel Strategy
- Digital Sales
Operations
Facilitating regulatory strategy to streamline European market entry and compliance process for medical devices.
- Project Management Office (PMO)
- Growth Strategy Implementation
- Operation Transformation
- Manufacturing
- Organization Design
Supply Chain Management
Optimize European healthcare delivery by enhancing medical device supply chains with innovative solutions.
- Supply Chain
- Digital Supply Chain
- Procurement
- Logistics
M&A, Transactions, and PMI
Certainly, please find the description crafted for this specific scenario below: ```json { "response": "Orchestrate seamless PMI for European medical devices via strategic M&A insights." }
- M&A Strategy
- Divesstiture/Curb-out Strategy
- M&A Target Scouting
- Buyer/Investor Search
- Commercial Due Diligence
- M&A Execution (Buy-side & Sell-side Financial Advisory)
- Valuation
- Post-Merger Integration (PMI)
- Value Creation
- IPO PMO
- Joint Venture
- Corporate Venture Capital
Strategy
Leverage YCP's strategic counsel to navigate Europe's medical device landscape with precision.
- Business Strategy
- Corporate Finance
- Growth Opportunity Identification
- New Business Development
- Organization Strategy
- Enterprise Risk Management
- Asset/Project Risk Management
- Digital Strategy
- Strategic Partnership & Channel Strategy
Digital Transformation
YCP drives actionable digital changes to optimize productivity for Europe's medical device firms.
- DX Vision
- Digital Roadmap
- CIO/CDO Support
- System Architecture Design
- DX PMO
- Artificial Intelligence (AI)
- Robotic Process Automation (RPA)
- Big Data Analysis
- IT Organization Design & Restructuring
- IT Portfolio Management
- Off-shore Development Centre Set-up
Public Services
Crafting strategic roadmaps that navigate European regulations for your medical device success.
- Economic Development
- Public Policy Development
- E-Governance
Sustainability
Navigating Europe's medical device regulations, YCP ensures sustainable compliance and growth.
- Sustainability Strategy & Implementation
- Double Materiality
- Decarbonization & Net-Zero Strategy
- Climate Change Risks and Opportunities
- Sustainability Due Diligence
- Supply Chain Assessment
- ESG Reporting
- Investor Relations & Fundraising
- Organization & Governance Design
Market Research
Globally connected, YCP deciphers European regulatory intricacies to elevate your medical devices' market entry.
- Market Landscape
- Market Size
- Industry Structure and Trend
- Competitive Benchmark
- Business Partner Search & Screening
- B2B/B2C Customer Survey
- Consumer Research
Navigating Medical Device Sector in Europe
Regulatory Compliance Complexity
- With EU Medical Device Regulations continually evolving, companies face a compliance headache. The sector must align with updated dossiers that include targeted electronic data gathering estimates of over 70%.
- Adopting a proactive stance, firms can utilize cost-effective audit software to maintain regulatory alignment, streamlining processes to achieve this at potentially half the traditional time frames.
High Market Entry Barriers
- Entering the European medical device market is daunting with certifications costs known to exceed €100,000 due to stringent CE mark requirements that may take upwards of two years.
- Leveraging local legal expertise and consulting services can mitigate these costs and optimize timelines by organizing dossiers tailored to specific EU frameworks consequently expediting market entry.
Increasing Demand for Health Tech Innovations
- The European medical device sector witnesses growing excitement, spiking at an annual rate of 7%, driving industry's appetite for adherence to innovative medical concerns and tools.
- Device manufacturers embracing these tech advancements can meet burgeoning EU market-driven needs with informed adoption contributing up to 15% boost in competitiveness and relevance.
Expanding Regional Cohesion Efforts
- The increasing medical harmonization within Europe encourages regional strategies, amplifying mutual gains as the sector sees projected synergies rising by 20% within organizational behaviors.
- By targeting regional collaborations businesses multiply impact elevating network advancements potential enhance projected synergetic outcomes for thriving regional initiatives supportifies effective expansion passage.
Our Clients
We have worked with more than 2000 companies throughout various industries.












What Our Clients Say About Us
"I would like to take this opportunity to express my sincere gratitude for all the effort and commitment that YCP showed us during this project. Although building detailed strategies for different countries within a tough time constraint is without any doubt a demanding and challenging task, YCP did a fabulous job. I believe that the success in this project laid a foundation for building a strong relationship between YCP and GS Caltex, and I promise that YCP will be the first one to be contacted whenever GSC needs any help for growth."

Manager, Finished Lubricants Marketing Strategy Team
GS Caltex
"YCP has proven to be a very solid partner. We enjoyed the regular project interactions enabling us to better leverage the market situation. The advisory work done by YCP was very useful to set our business direction and marketing investments. We also want to thank the YCP team for their professional approach and client management. It was a project worth our time and investment. "

Vice President, International Marketing Department
PTT Public Company
Results Through Expertise: Case Studies
Learn how we help clients build and implement strategies
that drive sustainable growth in today’s complex business landscape.
E-Commerce Website Development
Client
A Japanese medical equipment manufacturer
Area
Southeast Asia
Expertise Scope
Strategic Marketing
Project Summary
Our client needed support tapping into the Southeast Asian market and building an e-commerce channel. We created a market entry strategy in three countries, developed localised landing pages, and managed client campaigns to gain market access.
Acquisition Support for Medical Device Distributors in Southeast Asia
Client
A well-established European healthcare multinational company
Area
Southeast Asia
Expertise Scope
M&A Target Identification and Due Diligence, Market Entry Strategy
Project Summary
We assisted a European healthcare multinational in acquiring medical device distributors or OTC manufacturers across Southeast Asia. Over 12 months, we shortlisted top targets using strict criteria, initiated direct outreach, and facilitated NDA signings and meetings. A promising prospect in Singapore was identified, leading to a non-binding offer in due diligence. The client then expanded the project to the entire region.
YCP in Media











Our Group CEO Yuki Ishida and Managing Partner Karambir Anand shared insights at the ASEAN–Japan Young Business Leaders’ Summit.

At the Asian Power Summit 2025, our Director Harika G. led a session on Asia’s evolving energy mix.

Singapore Business Review featured Karambir Anand on Singapore’s expanding solar capacity and energy challenges.
Publications

Digital Health Transformation and Telemedicine 2.0 in Thailand
Thailand’s healthcare system is highly advanced, supported by strong public funding and a growing private sector. Digital health adoption (such as telemedicine, EHRs, and mobile health) is improving efficiency, access, and continuity of care, positioning the sector for sustained growth and innovation.

AI in Healthcare: Keeping the Patient at the Center of Innovation
AI is reshaping healthcare by improving efficiency, diagnostics, and personalized care amid rising costs and workforce shortages. Applications span prediction, telemedicine, diagnostics, and robotics across providers, insurers, and pharma. Long-term impact depends on responsible adoption, data quality, and strong governance.

India’s CRDMO Market: Building Momentum through Operational Excellence
India’s CRDMO market is growing rapidly, driven by China+1 strategies, funding access, and policy support. However, scale, cost pressure, and regulatory demands pose challenges. To compete globally, companies will depend on operational excellence, technology adoption, and building scalable, end-to-end capabilities.
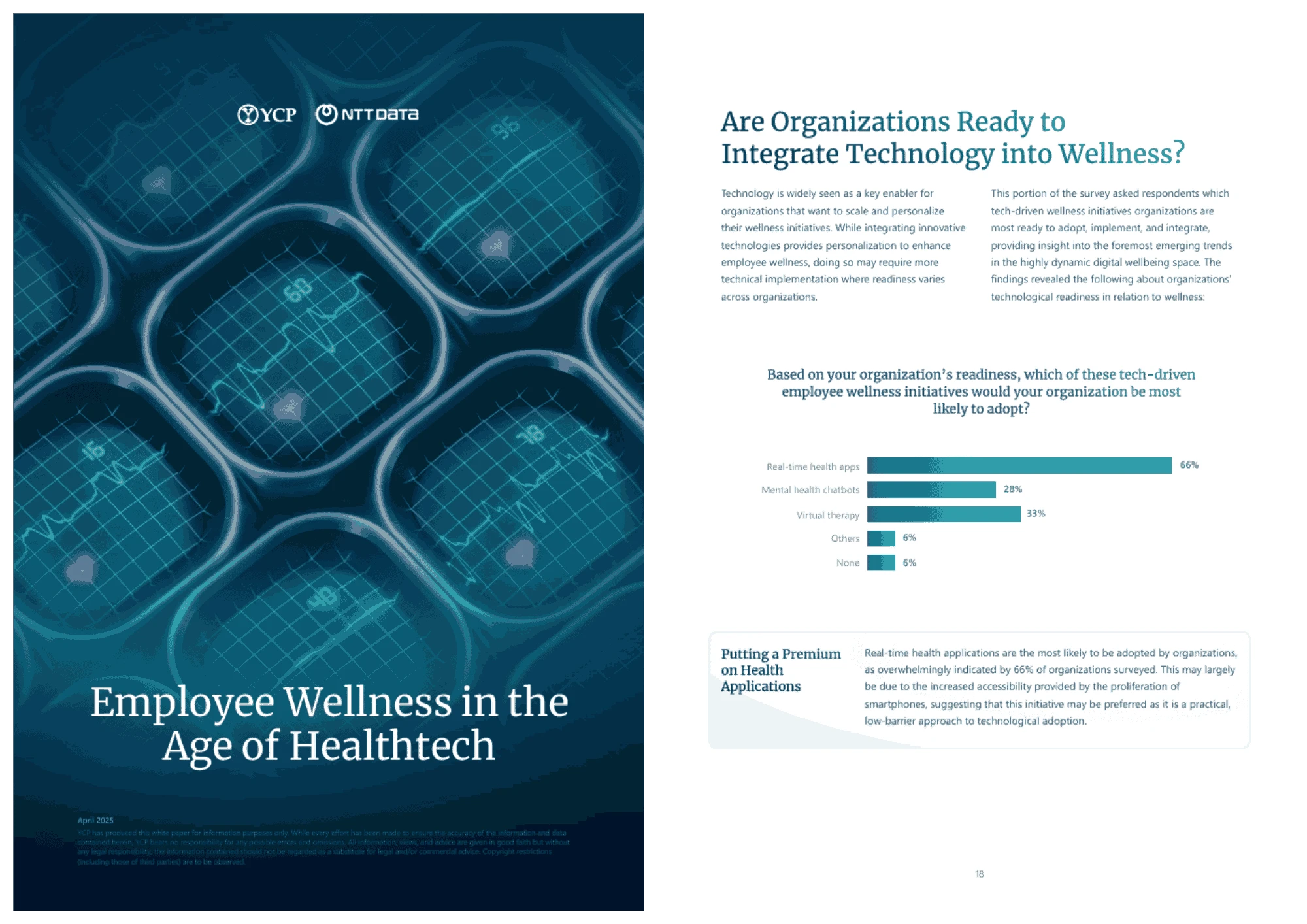
Employee Wellness in the Age of Healthtech
Employee wellness is becoming a strategic priority as workforce expectations evolve. Health technology is shifting programs toward personalized, proactive solutions, particularly across Asia. While data, privacy, and ROI challenges remain, organizations that integrate technology with human-centered design can build more resilient workforces.
Meet Our Experts
Establish industry dominance with our experts and partners

Pilar Dieter
Managing Partner
Nationality: American
Region Coverage:
Asia, North America
Past Experience:
Alaris Consulting

Jun Amano
Partner
Nationality: Japanese
Region Coverage:
Europe
Past Experience:
Dentsu Inc

Laurent Fihey
Partner
Nationality: French
Region Coverage:
Europe, Asia, Middle East, Africa, North America, South America
Past Experience:
Arthur Andersen, Advancy

Devshree Bhardwaj
Director
Nationality: Indian
Region Coverage:
Europe, North America, Australia, Asia, Middle East, South Africa, South America
Past Experience:
Tecnova India
YCP Group is the leading professional firm in Asia empowering excellence through diverse expertise:
- YCP
- YCP Auctus
- YCP Consus
Talk to Our Medical Device Experts
Arrange a session with our experts to explore how YCP can assist you in addressing your business challenges.
YCP Group is the leading professional firm in Asia empowering excellence through diverse expertise:
- YCP
- YCP Auctus
- YCP Consus


