Japan Pharmaceutical Manufacturing Consulting
Discover how YCP excels in Japan's pharmaceutical manufacturing sphere, guiding clients to success in this competitive market. YCP provides unparalleled support through decades of expertise, global reach, and a detailed-oriented approach, ensuring clients not only meet but exceed industry standards.

YCP in Numbers

Japan's pharmaceutical exports reached
USD 10.2 billion*
showing the sector's rapid international expansion in 2025.
Source: Japan Ministry of Finance, 2025
YCP Pharmaceutical Manufacturing Experts in Japan
The pharmaceutical manufacturing industry in Japan is experiencing remarkable growth, with advancements in various sectors. Japan's innovative spirit and dedication to quality make it a global leader, especially in the field of pharmaceutical manufacturing, driving progress and development across the nation.
At YCP, our seasoned global professionals offer exceptional pharmaceutical manufacturing consulting services. Our in-depth understanding of the industry's intricacies empowers clients to navigate market barriers and achieve successful market entry and growth. We've consistently delivered insightful solutions tailored to the unique challenges of the Japanese pharmaceutical market.
YCP stands as a trusted consulting partner for businesses seeking to thrive in Japan's pharmaceutical manufacturing arena. Our expertise and dedication to excellence enable clients to harness market potential confidently and effectively within the competitive landscape of Japan's drug development industry.
Unlocking Potential: Your Path to Excellence with
Japan's Pharmaceutical Manufacturing Professionals
Shopfloor Digitization
Leveraging deep expertise in Japan's Shopfloor Digitization, YCP optimizes pharmaceutical manufacturing through innovative tech integration.
Cycle Time Tracking
With unrivaled expertise in monitoring and improving cycle time tracking, YCP elevates pharmaceutical manufacturing processes amidst Japan's best practices.
Pharma Manufacturing Excellence
Leveraging unparalleled expertise, YCP optimizes pharmaceutical manufacturing efficiencies, ensuring success for Japanese pharmaceuticals in a competitive market.
Our Services
Marketing & Sales
Optimize your strategies in Japan, enhancing biomedical innovations for maximum market impact.
- Branding & Brand Management
- Product & Portfolio Management
- Pricing
- Digital Marketing
- E-commerce
- Customer Experience
- Sales Channel Strategy
- Digital Sales
Operations
Supporting streamlined operations, our consultancy offers innovative solutions in Japan's pharma sector.
- Project Management Office (PMO)
- Growth Strategy Implementation
- Operation Transformation
- Manufacturing
- Organization Design
Supply Chain Management
Navigating Japan's pharma landscape by optimizing supply chains for scalable manufacturing success.
- Supply Chain
- Digital Supply Chain
- Procurement
- Logistics
M&A, Transactions, and PMI
Facilitating seamless Pharmaceutical M&A and reversing maturation curves efficiently in Japan.
- M&A Strategy
- Divesstiture/Curb-out Strategy
- M&A Target Scouting
- Buyer/Investor Search
- Commercial Due Diligence
- M&A Execution (Buy-side & Sell-side Financial Advisory)
- Valuation
- Post-Merger Integration (PMI)
- Value Creation
- IPO PMO
- Joint Venture
- Corporate Venture Capital
Strategy
Redefine growth by navigating Japan's complexities with YCP's pharmaceutical manufacturing strategy.
- Business Strategy
- Corporate Finance
- Growth Opportunity Identification
- New Business Development
- Organization Strategy
- Enterprise Risk Management
- Asset/Project Risk Management
- Digital Strategy
- Strategic Partnership & Channel Strategy
Digital Transformation
Harness cutting-edge insights to revolutionize Japan's pharma manufacturing landscape.
- DX Vision
- Digital Roadmap
- CIO/CDO Support
- System Architecture Design
- DX PMO
- Artificial Intelligence (AI)
- Robotic Process Automation (RPA)
- Big Data Analysis
- IT Organization Design & Restructuring
- IT Portfolio Management
- Off-shore Development Centre Set-up
Public Services
YCP harnesses regulatory expertise to refine pharmaceutical operations in Japan efficiently.
- Economic Development
- Public Policy Development
- E-Governance
Sustainability
YCP strategically advises Japan's pharmaceutical firms on sustainable practices enhancing global competitiveness.
- Sustainability Strategy & Implementation
- Double Materiality
- Decarbonization & Net-Zero Strategy
- Climate Change Risks and Opportunities
- Sustainability Due Diligence
- Supply Chain Assessment
- ESG Reporting
- Investor Relations & Fundraising
- Organization & Governance Design
Market Research
Experiential analysis enhances Japan’s pharma manufacturing strategy for stronger market positioning.
- Market Landscape
- Market Size
- Industry Structure and Trend
- Competitive Benchmark
- Business Partner Search & Screening
- B2B/B2C Customer Survey
- Consumer Research
Navigating Pharmaceutical Manufacturing Sector in Japan
Rising Raw Material Costs Impact Forbes on Pharmaceutical
- The Japanese pharmaceutical manufacturing sector, worth $95 billion, is facing increasing raw material costs, which could drive up pricing and strain profit margins.
- In response, leveraging local production facilities and supply chains could help stabilize costs and ensure consistent manufacturing outputs.
Regulatory Hurdles Slowing Market Introduction
- Complex regulatory approval processes in Japan can take an average of three years, which delays the introduction of pharmaceutical innovations to market.
- Streamlining the regulatory process by adopting international GMP standards without compromising safety can accelerate the time-to-market for new drugs.
Aging Population Fuels Demand for Pharmaceuticals
- Japan’s citizens over 65 years make up 29% of the population, positively impacting demand for numerous pharmaceutical products and treatments.
- Pharmaceutical companies focusing on geriatric conditions and lifestyle disease therapies can meet growing needs with anticipated industry growth.
Growth in Biopharmaceuticals Spearheaded by Government Support
- The government's vision for biopharmaceutical expansion expects market growth rates of about 3% yearly due to increased investments.
- Active company participation in government-sponsored biotechnology initiatives reinforces strategic advantages in biopharma pursuits.
Our Clients
We have worked with more than 2000 companies throughout various industries.











What Our Clients Say About Us
"During the course of the process, I found YCP highly capable to support Toyota to better understand “ASEAN countries”. We usually work with Japanese consulting company and from their report, we can be a “book smart” but can’t have a clear picture of what’s going on in the real market. The project with YCP brought us “real market experience”. YCP's local consultants got their hands dirty in the marketplace with me. This approach enabled me to understand critical consumer insights behind data. I found it very valuable experience. Thank you very much."

Global Insights Manager
Toyota Motor Corporation
"I am very satisfied with YCP's work on competitors benchmarking analysis project in the Automotive Glass industry. The team fully understood the project background and objectives, and their output surely met our expectations. Although there were some challenges in collecting detailed information given this is a highly specialized industry, their analytical framework and logical approach made this project a great success. I also found the discussion with their local consultants very valuable. This gave me lots of useful insights to pursue the project. I would like to work with YCP on my next project. "

Senior Sales & Marketing Center Manager Automotive Glass Division
Asahi Glass Group
Results Through Expertise: Case Studies
Learn how we help clients build and implement strategies
that drive sustainable growth in today’s complex business landscape.
Business Transformation Strategy in the Japanese Healthcare Market
Client
A healthcare services support provider
Area
Japan
Expertise Scope
Budget Management, Operational Standardization, Customer Support Optimization
Project Summary
Our team managed cross-departmental budgets, tracked development progress, and streamlined the product planning process. We restructured business development teams, expanded call center operations, and created systems for customer support. To further enhance operations, we developed strategies for subscription services, implemented CRM and mobile solutions, and established a user-driven accounting system, setting the foundation for optimized organizational efficiency and improved client satisfaction.
PMO Support for a Biotechnology Firm in Japan
Client
A leading biotechnology firm based
Area
Japan
Expertise Scope
PMO Support, Stakeholder Mapping, Schedule Development
Project Summary
Our team provided PMO support in Japan, with tasks such as stakeholder mapping, creating schedules and task charts, preparing reimbursement dossiers, attending meetings with the Ministry of Health, Labour and Welfare (MHLW), and developing value story documents. This support was delivered by a partner-level team member and a seconded associate.
YCP in Media











Our Group CEO Yuki Ishida and Managing Partner Karambir Anand shared insights at the ASEAN–Japan Young Business Leaders’ Summit.

At the Asian Power Summit 2025, our Director Harika G. led a session on Asia’s evolving energy mix.

Singapore Business Review featured Karambir Anand on Singapore’s expanding solar capacity and energy challenges.
Publications

オペレーション診断に基づく 需要予測高度化の方向性と 経営貢献
不確実性の高い環境下では、需要予測はS&OPを支える戦略的なケイパビリティとなっています。予測精度は、システムやモデルの高度化だけでなく、プランナーのスキルや組織文化にも左右されます。診断フレームワークを活用することで、改善すべきポイントを的確に特定し、意思決定の俊敏性を高めることが可能となります。

AI in Healthcare: Keeping the Patient at the Center of Innovation
AI is reshaping healthcare by improving efficiency, diagnostics, and personalized care amid rising costs and workforce shortages. Applications span prediction, telemedicine, diagnostics, and robotics across providers, insurers, and pharma. Long-term impact depends on responsible adoption, data quality, and strong governance.

India’s CRDMO Market: Building Momentum through Operational Excellence
India’s CRDMO market is growing rapidly, driven by China+1 strategies, funding access, and policy support. However, scale, cost pressure, and regulatory demands pose challenges. To compete globally, companies will depend on operational excellence, technology adoption, and building scalable, end-to-end capabilities.
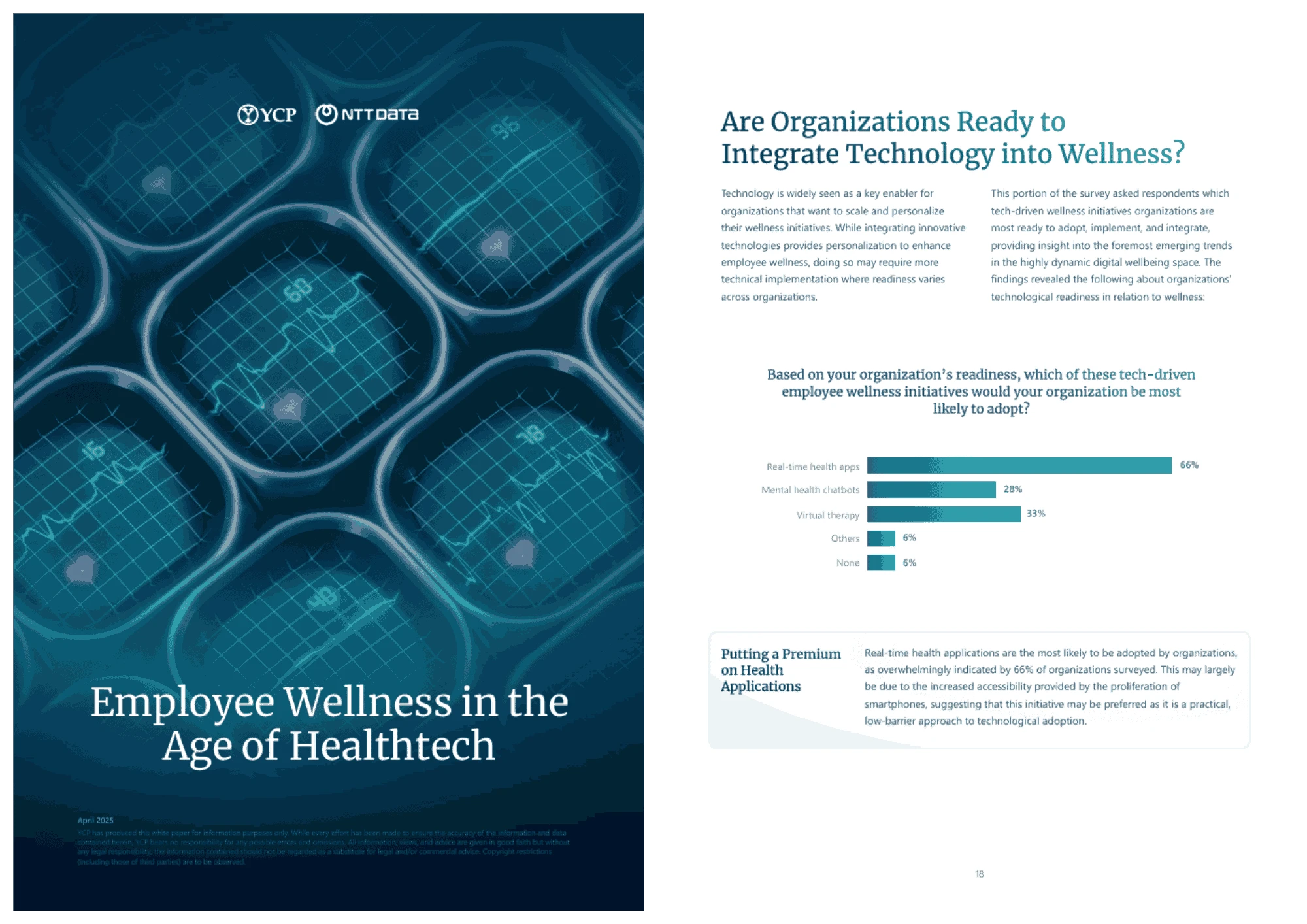
Employee Wellness in the Age of Healthtech
Employee wellness is becoming a strategic priority as workforce expectations evolve. Health technology is shifting programs toward personalized, proactive solutions, particularly across Asia. While data, privacy, and ROI challenges remain, organizations that integrate technology with human-centered design can build more resilient workforces.
Meet Our Experts
Establish industry dominance with our experts and partners

Yuki Ishida
Managing Partner
Nationality: Japanese
Region Coverage:
Asia, North America
Past Experience:
Goldman Sachs

Pilar Dieter
Managing Partner
Nationality: American
Region Coverage:
Asia, North America
Past Experience:
Alaris Consulting

Leon Cheng
Partner
Nationality: Chinese
Region Coverage:
Asia, North America, South America
Past Experience:
EAC, DHL

Mehdi Jaouadi
Partner
Nationality: French
Region Coverage:
Asia, Europe, South America
Past Experience:
METRO AG, L'Oréal

Shinjiro Sameshima
Partner
Nationality: Japanese
Region Coverage:
Asia
Past Experience:
UBS

Takanori Ono
Managing Partner
Nationality: Japanese
Region Coverage:
Asia
Past Experience:
Cisco, KPMG

Kana Iikura
Partner
Nationality: Japanese
Region Coverage:
Asia, Africa, Europe, North America
Past Experience:
KPMG AZSA

Masa Matsuoka
Managing Partner
Nationality: Japanese
Region Coverage:
Asia, Europe
Past Experience:
Nomura Research Institute, UBS Securities Japan
YCP Group is the leading professional firm in Asia empowering excellence through diverse expertise:
- YCP
- YCP Auctus
- YCP Consus
Talk to Our Pharmaceutical Manufacturing Experts
Arrange a session with our experts to explore how YCP can assist you in addressing your business challenges.
YCP Group is the leading professional firm in Asia empowering excellence through diverse expertise:
- YCP
- YCP Auctus
- YCP Consus


