United States Medical Device Manufacturing Consulting
YCP leads in United States Medical Device Manufacturing Consulting, offering strategic insights to thrive in the competitive market. Our global reach and hands-on approach enable clients to navigate challenges with confidence, leveraging YCP’s extensive experience and wide service range. Partner with YCP to harness growth opportunities in U.S. medical devices.

YCP in Numbers

YCP Medical Device Manufacturing Experts in United States
The medical device manufacturing industry in the United States is experiencing transformative growth, driven by technological advancements and innovative breakthroughs. The country is blooming across various sectors with significant progress in medical device manufacturing, fostering an environment ripe for further innovations.
YCP, with a vast network of global professionals and a profound grasp of the medical device manufacturing sector, offers unparalleled consulting services. Our expertise has been instrumental in helping clients navigate complex market barriers, ensuring seamless entry, and fostering growth within the United States' dynamic medical device manufacturing landscape.
As a trusted consulting partner, YCP empowers businesses to excel in the United States' competitive medical device manufacturing market. Our unique insights and strategic approach deliver impactful results, making YCP the go-to ally for sustainable success. Whether dealing with entry complexities or striving for growth, our clients consistently achieve their business objectives.
Unlocking Potential: Your Path to Excellence with
United States's Medical Device Manufacturing Professionals

Line Optimization
Championing efficiency, YCP's line optimization expertise in US medical device manufacturing helps maximize production, cut costs, and ensure compliance.

Line Optimization
In the rapidly evolving United States medical device manufacturing industry, YCP excels in line optimization, offering unparalleled consulting expertise.

Sterilization Process Redesign
With in-depth know-how in sterilization process redesign, YCP propels United States medical device manufacturing to new heights, ensuring compliance and innovation.

Quality Compliance
YCP excels in the intricate realm of Quality Compliance for U.S. medical devices, ensuring rigorous adherence to FDA regulations and fostering innovation.
Our Services
Marketing & Sales
Enhancing market reach and optimizing sales strategies for U.S. medical device leaders.
- Branding & Brand Management
- Product & Portfolio Management
- Pricing
- Digital Marketing
- E-commerce
- Customer Experience
- Sales Channel Strategy
- Digital Sales
Operations
Optimize production processes and regulatory compliance for medical device manufacturers in the U.S.
- Project Management Office (PMO)
- Growth Strategy Implementation
- Operation Transformation
- Manufacturing
- Organization Design
Supply Chain Management
YCP optimizes US medical device firms' supply chains with strategic consultation tools.
- Supply Chain
- Digital Supply Chain
- Procurement
- Logistics
M&A, Transactions, and PMI
Streamline M&A for transformative growth in US medical device manufacturing ventures.
- M&A Strategy
- Divesstiture/Curb-out Strategy
- M&A Target Scouting
- Buyer/Investor Search
- Commercial Due Diligence
- M&A Execution (Buy-side & Sell-side Financial Advisory)
- Valuation
- Post-Merger Integration (PMI)
- Value Creation
- IPO PMO
- Joint Venture
- Corporate Venture Capital
Strategy
YCP navigates complex regulations to drive streamlined innovation in U.S. medical device manufacturing.
- Business Strategy
- Corporate Finance
- Growth Opportunity Identification
- New Business Development
- Organization Strategy
- Enterprise Risk Management
- Asset/Project Risk Management
- Digital Strategy
- Strategic Partnership & Channel Strategy
Digital Transformation
YCP seamlessly integrates advanced digital solutions to revolutionize U.S. medical device manufacturing.
- DX Vision
- Digital Roadmap
- CIO/CDO Support
- System Architecture Design
- DX PMO
- Artificial Intelligence (AI)
- Robotic Process Automation (RPA)
- Big Data Analysis
- IT Organization Design & Restructuring
- IT Portfolio Management
- Off-shore Development Centre Set-up
Public Services
I'm going to need additional clarification about your request so I can meet your expectations accurately.
- Economic Development
- Public Policy Development
- E-Governance
Sustainability
We optimize operations and compliance with unparalleled insight into sustainable practices.
- Sustainability Strategy & Implementation
- Double Materiality
- Decarbonization & Net-Zero Strategy
- Climate Change Risks and Opportunities
- Sustainability Due Diligence
- Supply Chain Assessment
- ESG Reporting
- Investor Relations & Fundraising
- Organization & Governance Design
Market Research
We conduct precise analysis to navigate US medical device trends, ensuring informed decisions.
- Market Landscape
- Market Size
- Industry Structure and Trend
- Competitive Benchmark
- Business Partner Search & Screening
- B2B/B2C Customer Survey
- Consumer Research
Navigating Medical Device Manufacturing Sector in United States
Increased Compliance Regulations Expanding Costs
- The U.S. medical device sector now faces stringent compliance requiring an estimated 30% rise in budget allocation for certifications and audits.
- Given the complexities, adopting innovative compliance solutions can streamline operations, reducing failure risks and optimizing costs by 20% in the long term.
Bridging The Workforce Skill Gaps
- The sector's growth is stunted by a skilled labor shortage, with 25% positions unfilled by critical tech advancements in production demands.
- To fulfil this, upskilling existing talent to frontier technologies can swiftly convert personnel gaps, reflecting an increase in productive economies by 15%.
Rising Innovations Running Through Pipeline Projects
- The U.S. market accounts for 45% of worldwide R&D innovation in medical devices, showcasing dynamic tradewinds of novel gear introductions by sector locals.
- Leveraging unprecedented GlidePath initiatives could mark development succession spikes, boosting sector penetration efforts overflowing research rewards forecast.
Digital Technological Transformation Adeptness Environmental
- 80% of organizations now onboard essential courses via agile digital adaptation benefiting revolutionary scope benefits leading integration levels peaking post years.
- Tailored integration enhances dynamic targeting stimulations directed investments realizing revenue escalations consistent with local to global flow operands.
Our Clients
We have worked with more than 2000 companies throughout various industries.












What Our Clients Say About Us
"I would like to take this opportunity to express my sincere gratitude for all the effort and commitment that YCP showed us during this project. Although building detailed strategies for different countries within a tough time constraint is without any doubt a demanding and challenging task, YCP did a fabulous job. I believe that the success in this project laid a foundation for building a strong relationship between YCP and GS Caltex, and I promise that YCP will be the first one to be contacted whenever GSC needs any help for growth."

Manager, Finished Lubricants Marketing Strategy Team
GS Caltex
"YCP impressed me most with their ability to get to the truth on some very specific questions, bringing a level of concreteness to the content that I’ve never seen from another consulting firm. Their work provided valuable analysis for decision-making. The team at YCP is efficient, insightful and fearless!"

General Manager for APAC & Director of Global Logistics and Material Handling Business Unit
Intralox
Results Through Expertise: Case Studies
Learn how we help clients build and implement strategies
that drive sustainable growth in today’s complex business landscape.
Investor Meeting Arrangement for a Biotech Company and Strategic Partner
Client
A global biotechnology company enabling sustainable agriculture
Area
United States
Expertise Scope
Sustained Release Systems Development, Antibiotic Resistance Targeting, Product Line Expansion
Project Summary
The approach involved developing sustained release systems for the treatment and prevention of infections and targeting key topical antibiotic-resistant pathogens. Additionally, plans broadened to expand the product line to include treatments for diabetic ulcers, burn care, and wound care.
Capital Raising Support for Biotech Development in Infection Treatment
Client
A global biotech company
Area
United States
Expertise Scope
Capital Raising and Investor Relations
Project Summary
We assisted a biotech company focused on bacteriophage and lysine-based products in its efforts to raise $20 to $30 million for pre-clinical development, clinical trials, and a manufacturing facility. The company’s approach targets the treatment and prevention of infections caused by antibiotic-resistant pathogens through sustained release systems. They aim to expand their product line to include treatments for diabetic ulcers, burn care, and wound care.
YCP in Media











YCP Solidiance's Managing Partner Damien Duhamel talks on BBC on the Indonesian mining industry.

YCP Solidiance’s Partner Gervasius Samosir speaks to CNBC about the economy outlook after Indonesia’s presidential election.

Tagged as Asia’s Game Changer, YCP Holdings wins the Management Consulting award in SBR National Business Awards.
Publications

Investment Trends in Asia: How Trade Tensions Are Impacting Growth

AI in Healthcare Whitepaper

CRDMO Whitepaper
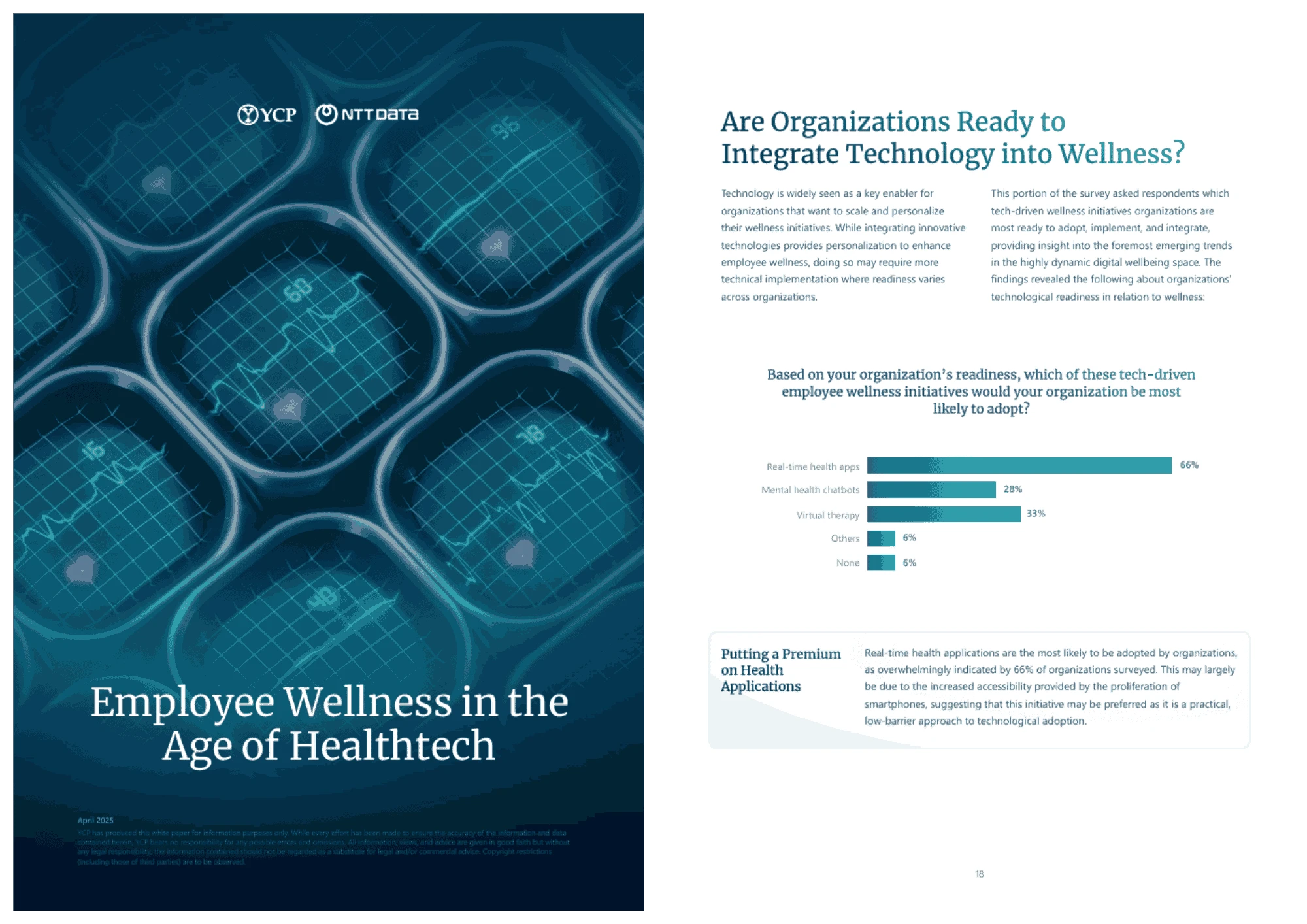
Employee Wellness in the Age of Healthtech
Meet Our Experts
Establish industry dominance with our experts and partners

Yuki Ishida
Managing Partner
Nationality: Japanese
Region Coverage:
Asia, North America
Past Experience:
Goldman Sachs

Pilar Dieter
Managing Partner
Nationality: American
Region Coverage:
Asia, North America
Past Experience:
Alaris Consulting

Jun Amano
Partner
Nationality: Japanese
Region Coverage:
Europe
Past Experience:
Dentsu Inc

Leon Cheng
Partner
Nationality: Chinese
Region Coverage:
Asia, North America, South America
Past Experience:
EAC, DHL

Saurabh Mehta
Managing Partner
Nationality: Indian
Region Coverage:
North America, Asia, Middle East
Past Experience:
Ivalua, Avon

Craig Morin
Partner
Nationality: American
Region Coverage:
Asia, North America, Europe, Middle East
Past Experience:
Tompkins Ventures, Adidas

Laurent Fihey
Partner
Nationality: French
Region Coverage:
Europe, Asia, Middle East, Africa, North America, South America
Past Experience:
Arthur Andersen, Advancy

Jason George
Partner
Nationality: American
Region Coverage:
North America
Past Experience:
Dalet, Charter Communications
Talk to Our Medical Device Manufacturing Experts
Arrange a session with our experts to explore how YCP can assist you in addressing your business challenges.
YCP Group is the leading professional firm in Asia empowering excellence through diverse expertise:
- YCP
- YCP Auctus
- YCP Consus
YCP Group is the leading professional firm in Asia empowering excellence through diverse expertise:
- YCP
- YCP Auctus
- YCP Consus

